Welcome to the 116th edition of the Research Digest! In this issue, we explore emerging blood-based biomarkers for ME/CFS and share findings from a study highlighting the high burden of chronic overlapping pain conditions and their impact on health and quality of life. From the long COVID field, new research links persistent spike protein in the brain to lasting neurological symptoms. We also share Sarah’s story – a personal account of how ME/CFS reshaped her life and career, and how support helped her find a new path forward.
Contributing Digesters: Jyothsna, Sarah, Solène and Simone.
We hope you enjoy the Easy Read Overview and audio summary we’ve included under each article’s title – it’s now even easier to stay informed of the latest ME/CFS and long COVID research!
You can also join our community and choose to have the Digest delivered straight to your inbox at the end of every month, by signing up to our mailing list here. We appreciate the support of everyone who reads the Digest – we encourage regular subscribers to support us with a monthly suggested donation of $2.
The search for a blood‑based biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): From biochemistry to electrophysiology
Authors: Clarke KSP, Kingdon CC, Hughes MP, Lacerda EM, Lewis R, Kruchek EJ, Dorey RA, and Labeed FH (United Arab Emirates University, UAE)
Publication: Journal of Translational Medicine
Link: https://doi.org/10.1186/s12967-025-06146-6
Easy Read Overview: Scientists are trying to find a simple blood test to help diagnose ME/CFS early, track how it changes, and choose the right treatments. They are studying changes in energy production, immune signals, and other body processes, but results are not always the same across studies. Even though no test works perfectly yet, using a mix of different methods might lead to better and more accurate ways to detect ME/CFS.
There is an urgent need to research a reliable blood-based biomarker that could enable early detection of ME/CFS, guide treatment, and track disease progression. Researchers in the present study have focused on considering common blood-based pathological changes in ME/CFS as potential biomarkers.
Mitochondrial dysfunction – There is considerable evidence of mitochondrial dysfunction and oxidative stress in ME/CFS, although findings have been inconsistant across studies. Some studies found reduced ATP production and oxidative phosphorylation in immune cells, while others did not. The diagnostic reliability of the “ATP Profile Test” (a commercially available laboratory test designed to measure mitochondrial function in neutrophils in patients with fatigue) remains uncertain, and studies using protein expression and metabolomics lacked specificity due to overlap with other diseases.
Kynurenine pathway – The kynurenine pathway is responsible for tryptophan metabolism. It is thought that this pathway may contribute to ME/CFS through a reduction in nicotinamide adenine dinucleotide (NAD+), which is an important source of cellular energy. The indolamine-2,3-dioxygenase (IDO) metabolic trap hypothesis suggested that there may be a genetic block in tryptophan conversion in ME/CFS. However, the results of these studies have been inconsistent and require further validation.
Itaconate shunt – In the presence of pathogens, the itaconate pathway downregulates ATP production, to reduce the ability of pathogens to access cellular energy for their own survival. The itaconate shunt hypothesis proposes that this mechanism doesn’t turn off in ME/CFS, resulting in ongoing impaired cellular energy production. The hypothesis suggests that the pathway remains activated only in specific cell types, such as macrophages, monocytes, and muscle cells.
Metabolomic and biochemical analyses – Metabolomic analysis using nuclear magnetic resonance (NMR) and mass spectrometry identified ME/CFS disease-specific metabolites, but their costly and complex nature limited clinical use. In contrast, Raman spectroscopy – a low-cost and rapid technique – demonstrated 91% diagnostic accuracy, although further validation is required.
Other potential biomarkers – Cytokines and chemokines – immune signalling proteins with altered profiles in ME/CFS patients – have also been investigated as potential biomarkers. However, results have varied significantly between studies. Differences in laboratory methods and disease variability have limited their value as reliable diagnostic biomarkers. Electrophysiological dysfunction, particularly abnormalities in acetylcholine receptors and transient receptor potential (TRP) channels, is emerging as a potential biomarker, offering further diagnostic leads.
The authors highlight the promise of various potential biomarkers, noting their specificity and reproducibility. While no single method has yet been successful, a multimodal approach may enhance sensitivity and specificity. The authors suggest that findings could be extrapolated to focus on refining techniques to ensure the development of reliable, accessible biomarkers for ME/CFS.
Chronic overlapping pain conditions in people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a sample from the Multi-site Clinical Assessment of ME/CFS (MCAM) study
Authors: Fall EA, Chen Y, Lin JMS, Issa A, Brimmer DJ, Bateman L … & Unger ER (Centers for Disease Control and Prevention, USA)
Publication: BioMed Central Neurology
Link: https://bmcneurol.biomedcentral.com/counter/pdf/10.1186/s12883-024-03872-0.pdf
Easy Read Overview: People with ME/CFS often have other painful conditions, called COPCs, like migraines and fibromyalgia. This study found that most people with ME/CFS had at least one of these conditions, which made their symptoms worse, especially for women. Managing these extra conditions may help people with ME/CFS feel better and improve their quality of life.
Patients with ME/CFS experience a wide array of symptoms, with pain being among the most common. Comorbid pain conditions may worsen the severity of symptoms and overall health status. Chronic overlapping pain conditions (COPC) are a group of pain related conditions that often occur together and may share related disease mechanisms. This study sought to identify comorbid COPC’s in ME/CFS patients and measure their impact on disease severity.
The data of 923 participants were included in this study (595 with ME/CFS, 328 healthy controls). Participants had been part of the Multi-Site Clinical Assessment of ME/CFS study (conducted at seven specialty American clinics from 2012-2020). Eight COPCs were included in this study: chronic low back pain, chronic migraine/headache, fibromyalgia, interstitial cystitis/irritable bladder, irritable bowel syndrome, temporomandibular disorder and endometriosis and vulvodynia. The presence of COPCs was determined by medical history and reports of symptoms for at least three months. Questionnaires were used to measure symptoms, functioning and illness severity.
The authors found that 76% of ME/CFS participants had at least one COPC, compared to 17.4% of healthy controls. Amongst the ME/CFS participants, chronic migraine/headache was most common (48.1%), followed by fibromyalgia (45.0%), chronic low back pain (33.1%), and irritable bowel syndrome (31.6%). With the exception of temporomandibular disorder, all COPCs were significantly more frequently seen in females than males. The COPCs of chronic low back pain and fibromyalgia each had a significant effect on health measures, especially in pain attributes. COPCs also had a significant impact on non-pain attributes and quality of life measures (such as post-exertional malaise, light sensitivity, sleep-related issues).
The authors found that COPCs were common in ME/CFS participants and that multiple COPCs further exacerbated the severity of illness, especially among female participants with ME/CFS. The authors conclude that assessment and management of COPCs may assist in improving health and quality of life for patients living with ME/CFS.
Persistence of spike protein at the skull-meninges-brain axis may contribute to the neurological sequelae of COVID-19
Authors: Rong Z, Mai H, Ebert G, Kapoor S, Puelles VG, Czogalla J,…, Ertürk A (Institute for Tissue Engineering and Regenerative Medicine, Germany)
Publication: Cell Host & Microbe
Link: https://doi.org/10.1016/j.chom.2024.11.007
Easy Read Overview:
Researchers found that the spike protein from COVID-19 can stay in the brain and may cause long-term problems. They discovered spike proteins in the skulls of mice and humans who had COVID-19, but not in people who never had it. Their experiments showed that the spike protein can cause brain inflammation and stress, which might explain why some people have lasting symptoms after infection.
SARS-CoV-2 infection can cause long-lasting neurological symptoms. The spike protein, one of the essential structural proteins of SARS-CoV-2 mediating its entry into the host’s cells, has been shown to persist in the skull-meninges-brain axis. This persistence may be involved in those neurological sequelae.
In this study, the authors used advanced optical tissue-clearing technologies to identify the tissue distribution of SARS-CoV-2 spike proteins. They used mice and human samples from four individuals (one died from COVID-19, three recovered from COVID-19 and died from another cause) and one individual control (who died before the SARS-CoV-2 pandemic).
They observed a spike protein accumulation in the skull-meninges-brain axis of all mice and post-mortem human samples but not the control sample. Mass spectrometry-based proteomics also revealed dysregulated neutrophil and inflammatory pathways in mouse and COVID-19 human samples.
Spike proteins were detected in the skulls of the three patients who recovered from COVID-19. In addition, neurodegeneration biomarkers were elevated in the cerebrospinal fluid of patients who showed long-term symptoms. Further experiments in healthy mice showed that spike proteins injected into the skull marrow induced stress and inflammation in the brain parenchyma. Spike proteins, when injected peripherally, triggered neuroinflammation and anxiety-like behaviour. Finally, exposure to spike proteins exacerbated the effects of cerebral ischemia and traumatic brain injury (TBI) in mouse models. Vaccination against COVID-19 reduced spike protein accumulation after SARS-CoV-2 infection in mice.
Overall, these findings suggest persistent spike protein in the brain may contribute to lasting neurological sequelae of COVID-19. Further investigation is needed to elucidate the impact of the spike protein on the brain.
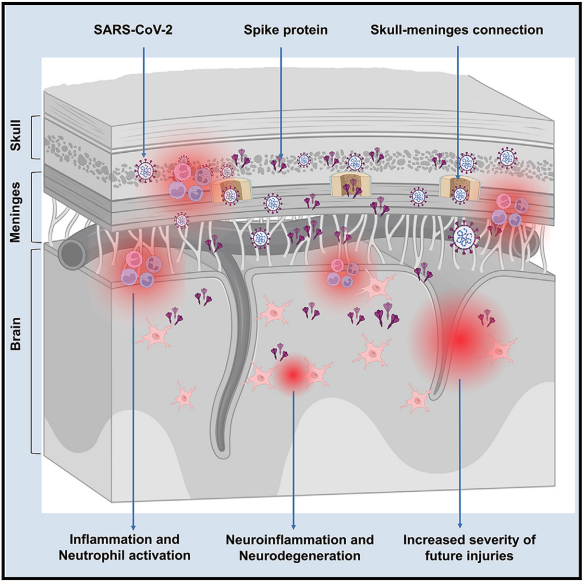
Figure. Graphical representation of research findings showing that the SARS-CoV-2 spike protein persists in the skull-meninges-brain axis, inducing inflammation, neurodegeneration-related changes, and increasing the brain’s vulnerability to further injury.
When illness forced Sarah out of work, she thought she'd be off for six months. It turned into eight years
Authors: Gleeson, H.
Publication: ABC News
Link: https://www.abc.net.au/news/2025-04-07/career-change-choice-me-cfs-chronic-illness-meander-valley/104847108
This article describes the experience of Sarah Williams, who left her job as an intranet designer at Melbourne University after ME/CFS made it impossible for her to keep working. “I felt so utterly sick and depleted that I knew I needed time off to rest completely”. She moved back to her family’s property expecting a short recovery, but “It turned into an eight-year stay.” With her mum becoming her carer, Sarah described how “life became very small,” as she dealt with the isolating effects of the illness.
The article highlights the difficulty maintaining employment for people with chronic illness. A report from Jobs Mobility Australia, which found that 6% of Australians lost a job because of ill-health or injury. Emerge Australia’s own survey of 1000 people with ME/CFS in Australia found that 89% had stopped work or reduced their hours due to their illness, and that two thirds of respondents were living below the poverty line.
Sarah describes how, with support from Emerge Australia, she eventually found a new direction. She renovated her grandfather’s cedar-shingled guesthouse and created a short-stay business, Cedar Cottage Meander. She explained, “I wanted to create a beautiful, restful place for people to come and recharge.”


